Common Processes For Amusement Park Equipment Rides – What Is The Heat Treatment?
Basic knowledge of metallurgy and heat treatment
(1) The crystal structure of metals is composed of atoms. According to the different arrangements of atoms in matter, matter can be divided into two categories: crystalline and amorphous. Any substance whose internal atoms are regularly arranged is called a crystal, and all substances whose internal atoms are irregularly arranged are called amorphous, and all solid metals are crystals.
The arrangement of atoms inside a crystal is called the crystal structure. Common crystal structures include body-centered cubic, face-centered cubic and hexagonal close-packed, as shown in Figure 2-1.
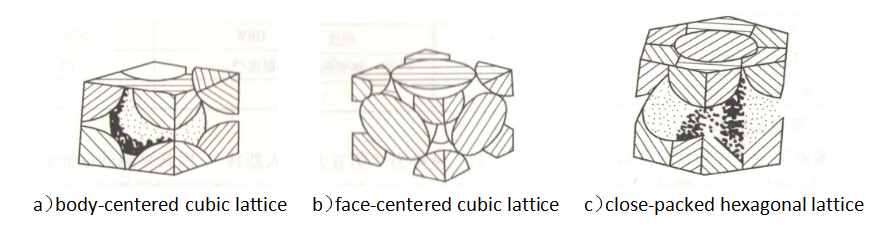
Figure 2-1 Schematic diagram of three typical lattices
Materials with different crystal structures have different characteristics: metals with body-centered cubic lattices have higher strength, hardness, and melting point, but poor plasticity and toughness; metals with face-centered cubic lattices have better plasticity and toughness. Cold brittleness; metals with a close-packed hexagonal lattice have low strength and poor toughness, and are generally not used as structural materials.
Metals are made up of many grains called polycrystals. Each grain is equivalent to a single crystal, and the atomic arrangement in the grain is the same, but the orientation of the atomic arrangement of different grains is different. The interfaces between grains are called grain boundaries.
The process of cooling liquid metal at high temperature into solid metal is a crystallization process, that is, the process of atoms transitioning from an irregular state (liquid state) to a regular state (solid state).
The atomic arrangement of the actual crystal is not perfect. Due to various reasons, the atomic arrangement of many parts of the crystal is damaged. The alternative atoms present in the metal host atoms are called impurities; impurities and the wrong arrangement of atoms in the crystal are collectively called “defects”, including There are four types of point defects, line defects, surface defects, and volume defects. Common crystal defects include vacancies, interstitial atoms, substitution atoms, and dislocations.
(2) The structure and chemical composition of the iron-carbon alloy are uniform, and there is an obvious interface separated from the surrounding area called the phase. Different phases have completely different structures and properties. Its arrangement and combination have various characteristic microstructures.
Steel is composed of multiphase microstructure, and its physical, chemical properties, and mechanical properties depend to a large extent on the type, proportion, size and spatial distribution of the phases.
Steel and cast iron are usually collectively referred to as iron-carbon alloys, because although the composition of steel and cast iron is complex, they are basically composed of two elements, iron and carbon. Generally, those with a carbon content of 0.02% to 2% are called steel, and those with a carbon content greater than 2% are called cast iron.
The relevant properties of steel can be analyzed through the Fe-Fe3C diagram (see Figure 2-2). Iron undergoes an allotropic transformation, that is, it has a different structure in the solid state. Different structures of iron and carbon can form different solid solutions, and the solid solutions on the Fe-Fe3C phase diagram are all interstitial solid solutions.
1)Amusement Park Equipment Rides Basic phases in iron-carbon alloys:
① Ferrite (F): A solid solution of carbon dissolved in α iron or δ iron (a solid solution refers to a single homogeneous substance formed by mutual dissolution of two or more elements that make up the alloy). Both alpha iron or delta iron are body-centered cubic lattices, the former refers to iron with a temperature below 910 °C, and the latter refers to iron with a temperature of 1390 to 1535 °C.
The ability of ferrite to dissolve carbon is extremely poor, and it is only 0.0218% when the amount of carbon dissolved at 727 °C is the largest. The strength and hardness of ferrite are not high, and it has good plasticity and toughness. It has ferromagnetism below 770℃, and loses ferromagnetism when it exceeds 770℃.
②Austenite (A): A solid solution in which carbon is dissolved in γ-iron. Gamma iron is a face-centered cubic lattice, and the austenite has a large carbon-dissolving ability, up to 2.11% (1148 ℃), and the dissolved carbon content at 727 ℃ is 0.77%. Austenite is not ferromagnetic.
③ Cementite (Fe3C): a metal compound of iron and carbon, its carbon content is 6.69%. The hardness of cementite is very high, while the plasticity and toughness are almost zero, and the brittleness is very large.
The cementite has weak magnetism at low temperature, and the magnetism disappears above 217 °C. Cementite is divided into three types: primary cementite, secondary cementite and tertiary cementite.
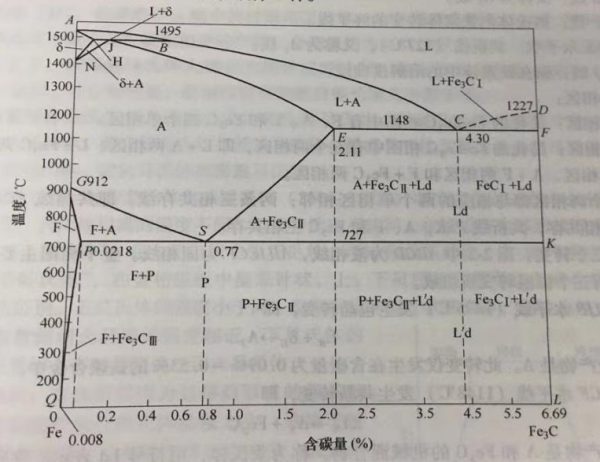
Carbon content(%)
Figure 2-2 Fe-Fe3C phase diagram
2) Analysis of Fe-Fe3C phase diagram:
①Main characteristic points: see Table 2-8.
Table 2-8 Characteristic points of Fe-Fe3C phase diagram
| Characteristic point symbol | Temperature/℃ | Carbon content(%) | Meaning |
| A | 1538 | 0 | Melting point: melting point of pure iron |
| C | 1148 | 4.3 | Eutectic point: eutectic transition occurs 14.30%→Ld (A2.11%+Fe3C eutectic) |
| D | 1227 | 6.69 | Melting point: the melting point of cementite |
| E | 1148 | 2.11 | Maximum solubility point of carbon in gamma iron |
| G | 912 | 0 | Allotropic transition point |
| S | 727 | 0.77 | Eutectoid point: eutectoid transformation A0.77%→P (P0.0218%+Fe3C eutectoid) |
| P | 727 | 0.0218 | Maximum solubility point of carbon in alpha iron |
| Q | Greenhouse | 0.0008 | Solubility of carbon in alpha iron at room temperature |
②Main characteristic line:
BC line: the starting line of the liquid to austenite transformation, that is, L—→A.
CD line: the starting line of the liquid to cementite transformation, that is, L—→Fe3C1. The ABCD line is collectively referred to as the liquidus line, and the alloy above this line is all in the liquid phase, which is represented by the symbol L.
JE line: The final line of liquid to austenite transformation.
ECF horizontal lines: Eutectic lines. The AHJECF line is collectively referred to as the solidus line. The liquid alloy is cooled until this line is completely crystallized into a solid, and the solidus region is below this line.
A→Fp+Fe3C
ES line: also known as Acm line, is the solubility curve of carbon in austenite, that is, L—→Fe3CII.
GS line: Also known as A3 line.
GP line: The final line of the transformation from austenite to ferrite.
PSK horizontal line: eutectoid line (727 ℃), also known as A1 line.
PQ line: Solubility curve of carbon in ferrite.
③ Amusement Equipment Phase area:
Single-phase region: There are four single-phase regions of F, A, L and Fe3C in the simplified Fe-Fe3C phase diagram.
Two-phase region: There are five two-phase regions in the simplified Fe-Fe3C phase diagram, namely L+A two-phase region, L+Fe3C two-phase region, A+Fe3C two-phase region, A+F two-phase region and F+ Fe3C two-phase region.
Each two-phase region is adjacent to the corresponding two single-phase regions; two three-phase coexistence lines, namely eutectic line ECF, L, A and Fe3C three-phase coexistence, eutectic line PSK, A, F and Fe3C three-phase coexistence coexist.
④Three transitions: ABCD is the liquidus line in Figure 2-2, and AHJECF is the solidus line. The whole phase diagram is mainly composed of three isothermal transitions: peritectic, eutectic and eutectoid.
The peritectic transition occurs at the HJB horizontal line (1495°C), i.e.
LB+δH→AJ
The transformation product is A. This transformation only occurs in iron-carbon alloys with a carbon content of 0.09% to 0.53%. The eutectic transition occurs at the ECF horizontal line (1148°C), i.e.
Lc→AE+Fe3C
The transformation product is a mechanical mixture of A and Fe3C, called ledeburite, denoted by the symbol Ld. This transformation occurs in iron-carbon alloys with carbon content ranging from 2.11% to 6.69%.
The eutectoid transition occurs at the PSK horizontal line (727°C), i.e.
A→Fp+Fe3C
The transformation product is a mechanical mixture of F and Fe3C, called pearlite, denoted by the symbol P. This transformation occurs in all iron-carbon alloys containing more than 0.0218% carbon. The eutectoid transition temperature is often referred to as the A1 temperature. In addition, there are three important solid-state transition lines in the Fe-Fe3C phase diagram:
1) GS line: F begins to precipitate in A, which is often called the A3 temperature.
2) ES line: the solubility line of carbon in A, often referred to as the Acm temperature. When the temperature is lower than this temperature, Fe3C will be precipitated in A, which is called secondary cementite Fe3CII, which is different from the primary cementite Fe3CI crystallized from the liquid through the CD line.
3) PQ line: the solubility line of carbon in F. When F is cooled down from 727 °C, Fe3C will also be precipitated, which is called tertiary cementite Fe3CIII.
(3) Common metallographic structure and properties of steel for special equipment Ferrite (F), austenite (A) and cementite (Fe3C) are common metallographic structures of steel for special equipment. Phase organization:
①Pearlite (P): A mixture of ferrite and cementite, which is the product of eutectoid transformation of carbon steel with a carbon content of 0.77%. The metallographic structure is a mechanical structure composed of lamellar ferrite and cementite. mixture. Pearlite has higher hardness and strength, and better plasticity.
The iron-carbon alloy with a carbon content of 0.77% only undergoes eutectoid transformation, and its structure is 100% pearlite, which is called eutectoid steel. Iron-carbon alloys with carbon content greater than 0.77% are called hypereutectoid steels, and their organization is pearlite P+ cementite Fe3C; iron-carbon alloys with carbon content less than 0.77% are called hypoeutectoid steels, and their organization is ferritic Body F + pearlite P.
Low carbon steel is hypoeutectoid steel, so under slow cooling conditions, the normal structure of low carbon steel is ferrite F + pearlite P. The lower the carbon content, the more ferrite content in the structure, the better the plasticity and toughness, but the lower strength and hardness.



② Martensite (M): It is a supersaturated solid solution of carbon dissolved in iron. It is the product of non-diffusion transformation after the steel is austenitized at high temperature and rapidly cooled to below the martensitic point.
The metallographic structure is a white needle-like structure with a certain angle to each other. Under the normal quenching process, most of the obtained martensite is fine needle or crypto needle. Martensite has high hardness (640~760HBW), is very brittle, has poor impact toughness, and the area shrinkage and elongation after fracture are almost equal to zero.
According to the carbon content, martensite can be divided into high carbon martensite and low carbon martensite. High carbon martensite is also known as flaky (acicular) martensite or twinned martensite, prismatic martensite and low temperature martensite; low carbon martensite is also known as lath martensite or Dislocation martensite, bulk martensite, directional martensite and high temperature martensite.
③Bainite (B): The supersaturated ferrite and cementite mixture produced by the transformation of supercooled austenite in the medium temperature range (250~450℃). The temperature at which bainite is formed is different, and the microstructure characteristics are also different.
The structure generated near the pearlite formation temperature is called “upper bainite”, which is feathery in the metallographic structure and can be symmetrical or asymmetrical. The structure formed near 300 °C is called “lower bainite”, and it is black needle-like in the metallographic structure.
The upper and lower bainite are only different in shape and carbide distribution, but have no qualitative difference. The strength of the upper bainite is lower than that of the flaky pearlite formed at the same temperature, and the brittleness is also greater.
Lower bainite has similar strength to tempered martensite at the same temperature, and the cooling, heat preservation and heating performance of lower bainite is better than that of upper bainite, and sometimes even better than tempered martensite.
④Weidmansten structure: coarse-grained austenite formed by hypoeutectoid steel due to overheating. The generation of Widmanderin is related to the austenite grain size (depending on the degree of superheating), the carbon content of the steel, the cooling rate and other factors.
When the widmanderin structure is serious, the impact toughness, the reduction of area and the elongation after fracture of the steel will decrease, and the steel will become brittle. It can be eliminated by full annealing.



2.General process of heat treatment
In the actual production process, the heat treatment process is relatively complicated. Its basic process consists of three stages of heating, heat preservation and cooling. Temperature and time are the main factors affecting the heat treatment. Any heat treatment process can be described by a temperature-time curve. Figure 2-3 shows the basic process of heat treatment.
It can be seen from the Fe-Fe3C phase diagram that with the change of temperature, the steel can undergo phase transformation in the solid state. Figure 2-4 shows the solid-state part of the steel in the iron-carbon Fe-Fe3C phase diagram.
The solid-state phase transition temperatures associated with low-carbon steel are the GS line and the PSK line, which are called the A3 line and the A1 line, respectively. During actual heating and cooling, due to the phenomenon of overheating and undercooling, the phase transition temperature of the steel during heating will be higher than the A3 line and the A1 line,
so the critical point of phase transformation during heating is represented by the Ac3 line and the Ac1 line; The phase transition temperature will be lower than the A3 line and the A1 line, so the critical point of phase transition during cooling is represented by the Ar3 line and the Ar1 line. The structural change of steel during heat treatment consists of several processes: first, when heated, the normal temperature structure of the steel is transformed into austenite;
second, the reason why the steel needs a holding time after heating is not only to burn the workpiece through, but also To make the core reach the same temperature as the surface, in order to obtain austenite structure with uniform composition, so as to obtain good structure and properties after cooling; third, austenite is decomposed during cooling, and different cooling rates are obtained with different cooling rates. Transformation products such as pearlite, ferrite or martensite of morphological components.


3.Commonly used heat treatment process
According to the change law of the structure and properties of steel during heating and cooling, the heat treatment process is divided into annealing, normalizing, quenching, tempering, etc.
Annealing
The heat treatment process in which the steel specimen is heated to an appropriate temperature, and then slowly cooled after holding for a certain period of time to obtain a structure close to the equilibrium state is called annealing. According to the composition and purpose of the steel, annealing is divided into complete annealing, incomplete annealing, stress relief annealing, isothermal annealing, spheroidizing annealing, etc.
1) Complete annealing is also called recrystallization annealing. The method is to heat the workpiece to 30~50°C above Ac3, and then cool it slowly in the furnace after heat preservation. Its purpose is to homogenize the structure, eliminate stress, reduce hardness and improve cutting performance.
2) Incomplete annealing is to heat the workpiece to 30~50°C above Ac1, and cool it slowly after heat preservation. Its purpose is to reduce hardness, improve cutting performance and eliminate internal stress.
3) The heating temperature of stress relief annealing varies with different materials. Generally, the workpiece is heated to 100~200°C below Ac1, and approximately 500~650°C for carbon steel and low-alloy steel, and then slowly cooled. Its purpose is to eliminate the internal stress caused by welding, cold deformation processing, casting, forging and other processing methods, and at the same time, it can make the hydrogen in the weld diffuse more completely, improve the crack resistance and toughness of the weld, and also improve the welding performance. It also plays a role in stabilizing the structure of the seam and the organization of the heat-affected zone.
(2) Normalizing
Normalizing is a heat treatment process in which the workpiece is heated to Ac3 or Ac1, above 30~50 °C, and cooled in air after a certain period of time. The purpose of normalizing is basically the same as that of annealing, mainly to refine grains, uniform structure and reduce internal stress. The difference between normalizing and annealing is that the former has a faster cooling rate and a larger degree of supercooling, which increases the amount of pearlite in the tissue and reduces the thickness of the pearlite lamellae. The strength, hardness and toughness of steel after normalizing are higher than those after annealing.
(3) Quenching
Quenching is a process in which the steel is heated above the critical temperature, and then rapidly cooled after proper heat preservation to transform austenite into martensite. The martensite structure of the material is obtained by quenching, which can improve its hardness and strength, which is beneficial to workpieces such as bearings and molds; but martensite is hard and brittle, with poor toughness and large internal stress, which is prone to cracks.
(4) Tempering
Tempering is a heat treatment process in which the quenched steel is heated to an appropriate temperature below Acg, kept for a certain period of time, and then cooled (usually air-cooled) by a method that meets the requirements to obtain the desired structure and properties. The main purpose of tempering is to reduce the internal stress of the material and improve the toughness.
By adjusting the tempering temperature, different hardness, strength and toughness can be obtained to meet the required mechanical properties. In addition, tempering stabilizes part dimensions and improves processability. According to the different tempering temperature, tempering can be divided into three types: low temperature tempering, medium temperature tempering and high temperature tempering. Tempered martensite is mainly used for tools and ball bearings made of various high carbon steels;
tempering in the range of 350~500℃ after quenching is called medium temperature tempering, and the structure after tempering is tempering bracket Tensite, mainly used in molds, springs, etc.; tempering in the range of 500~650 °C after quenching is called high temperature tempering, and the structure after tempering is tempered sorbite, which is characterized by a certain strength and at the same time.
It has high plasticity and impact toughness, that is, it has good comprehensive mechanical properties. The heat treatment of quenching and high temperature tempering is also called quenching and tempering. Many mechanical parts such as gears and crankshafts need to be quenched and tempered.
(5) Quenching and tempering
The heat treatment process that combines quenching and high temperature tempering is usually called quenching and tempering, or quenching and tempering for short. After quenching and tempering, the tempered sorbite structure can be obtained, which can make the steel part obtain good comprehensive performance in combination with strength and toughness.
Compared with normalizing, under the same hardness, the strength, plasticity and toughness of quenched and tempered steel are obviously improved compared with normalizing.





